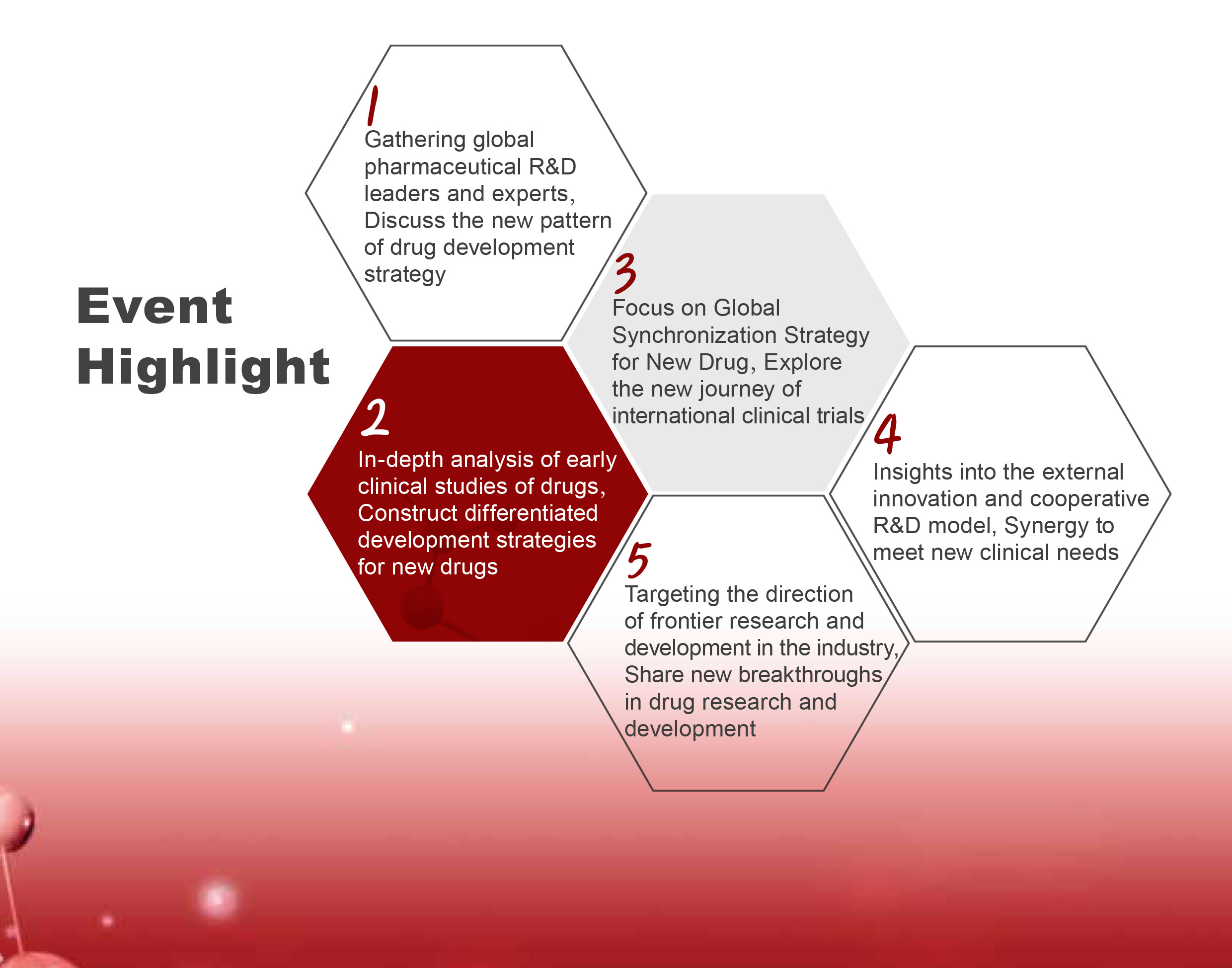
【Event Highlight】
【Hot Topics / Agenda】
>>Oriented by Clinical Value: The Science Road of China's Drug Development and
Regulatory
>>Globalization of New Drug Development: The Policies and Regulatory Environment
of Overseas Drug Review and Approval
>>Strategies and Key Challenges for
Early Research and Development of New Drugs
>>Source Innovation: Target Development
and New Compound Screening
>>Global Clinical Cooperation: The
Construction and Practice of International
Multi-Center Clinical Strategy
>>Key Elements and Considerations of
Innovative Drug Clinical Trial Design
>>Application of Translational Medicine
in the Early Research of New Drugs
>>] From Chinese New to Global New:
Construction of China's Pharmaceutical
Innovation Ecosystem
>>Thoughts on Regulatory Registration
and Risk Management under the Simultaneous
Development of Global New Drugs
>>Experience Exchange: Dilemma of Global
Clinical Trials and Practice Case Sharing
>>Analysis of the Key and Difficult Points
in the Development of Bispecific Antibody Drugs
>>The Next 100 Billion Market:
The Development of ADC Drugs
>>Strategy and Case Analysis of Small
Molecule Drug Development
>>CMC Key Strategies and Considerations in
the Development of New Small Molecule Drugs
>>Race of New Crown 'Specific Drugs':
Progress and Breakthroughs in Research and
Development of Neutralizing Antibody Therapeutic
Drugs
>>The Pattern of Antibody Research and
Development : Differentiated Development and
Layout of Antibody Drugs
>>Discussion on the High-Quality
Transformation Strategy of Innovative Drug
Commercialization
>>Competition in the Global Arena:
Differentiated Strategy and Layout of Small
Molecule Drug Research and Development
>>Strategy Sharing of Cell and Gene
Therapy Product Process Development
>>Development Progress of China's mRNA
COVID-19 Vaccine
>>Development and Innovative
Exploration of New AAV Gene Therapy
>>The New Pattern and Trend of Investment
and Mergers in China's Pharmaceutical Industry
>>Investment and Financing of Innovative
Pharmaceutical Companies: Strategies and
Considerations for Multi-Party Cooperation
>>Multinational Pharmaceutical Companies in
China: Drug R&D Model and Innovation Ecosystem
>>Road to Industrialization: Analysis of
Commercialization Strategy of Cell Therapy
>>Collaborative Innovation: Construction of
External R&D Innovation Model and Case Sharing
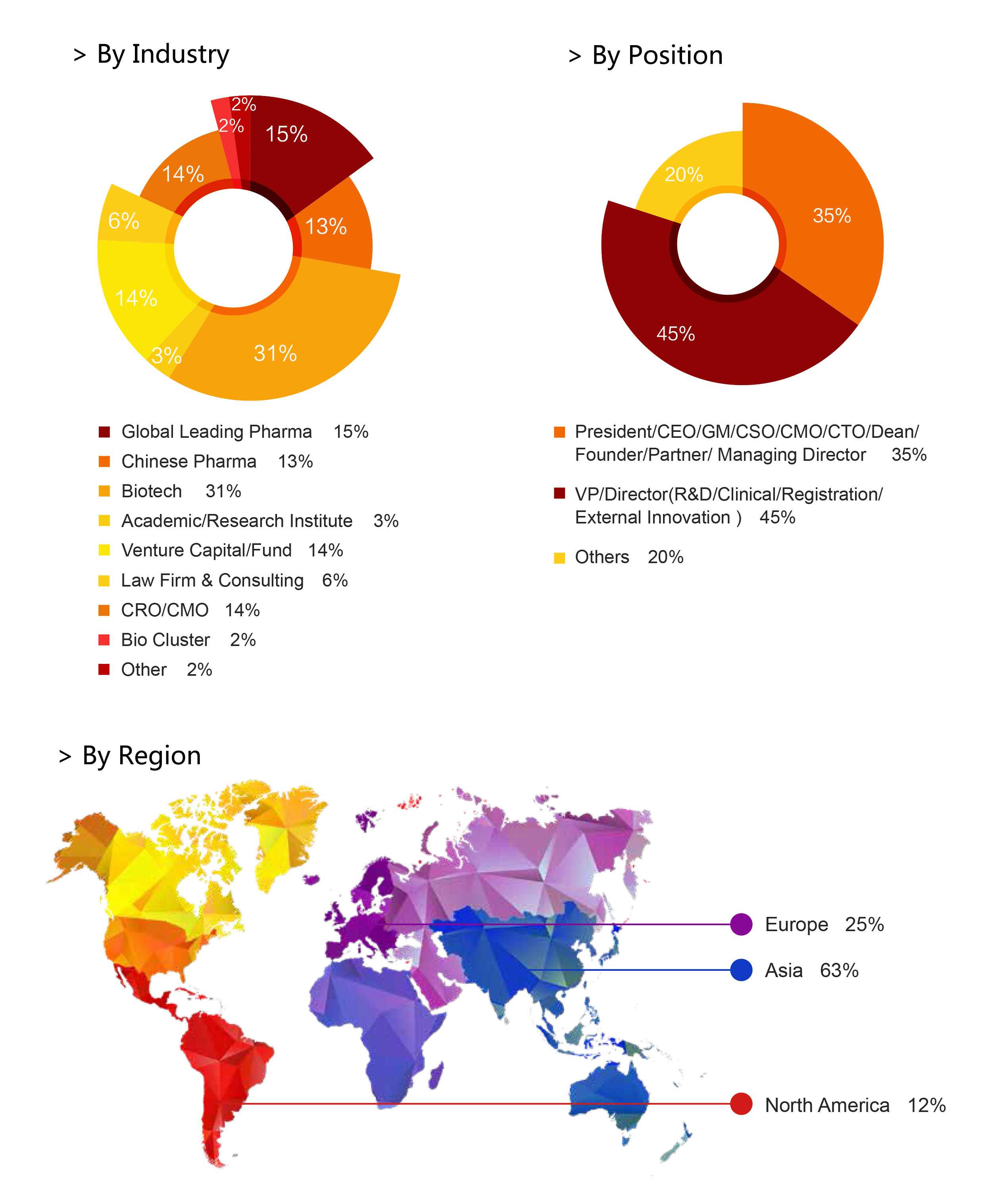
【Who should attend】
【Outstanding Past Speakers】



【Part of Previous Attending Companies】


【Wonderful Moment】
